
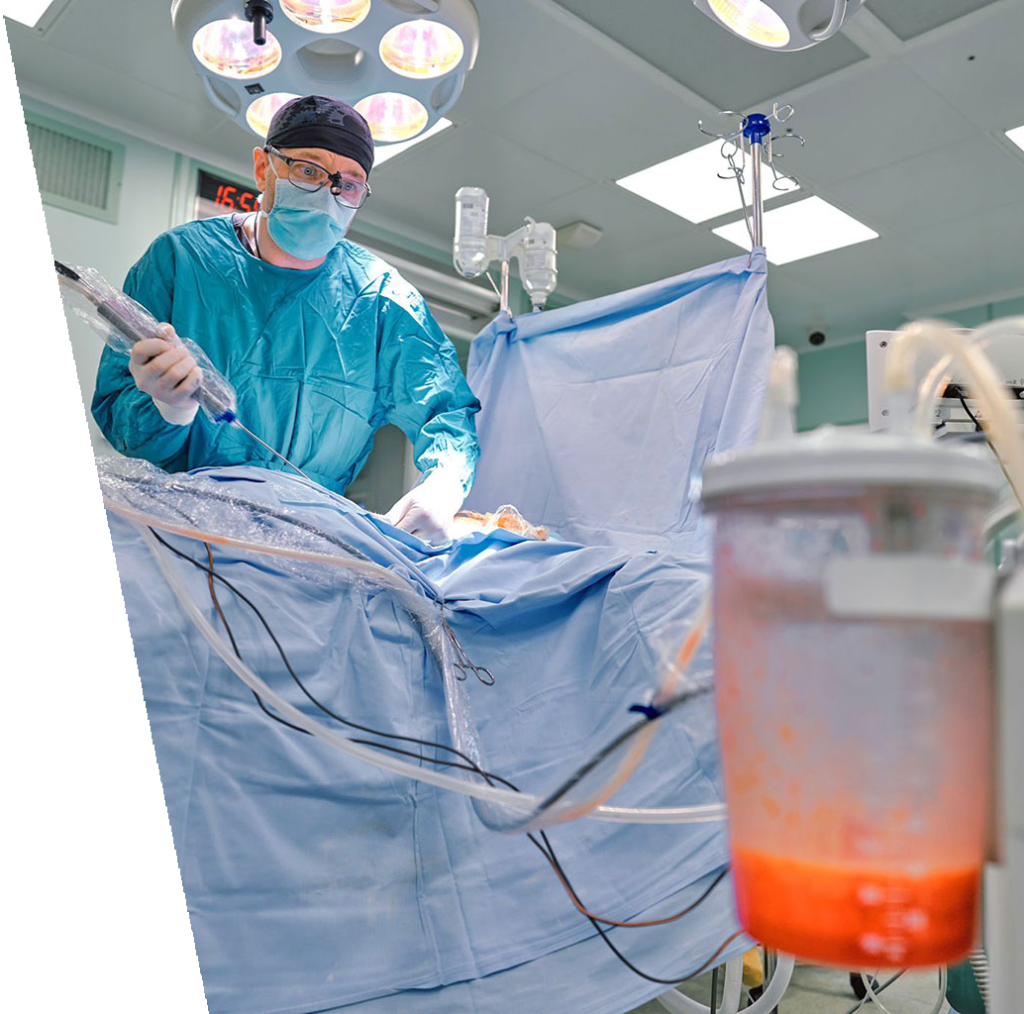
As the largest source of regenerative cells in the body, Fat (adipose tissue) which is typically discarded, presents a universal opportunity for an ever-expanding multitude of applications in:
Wrinkle Reduction
As a natural alternative to synthetic injectables, fat grafting for wrinkle reduction is expected to capture an increasing share of the market and contribute to the projected $21B anti-wrinkle market by 2030.
Blemishes
As consumers continue to prioritize natural skincare, the demand for blemish treatments using organic ingredients, such as fat, is poised to capture a significant share of a market that is expected to grow to $17.40 B by 2028.
Scar Reduction
Fat grafting is rapidly emerging as a leading option for scar reduction, offering a natural and effective solution, which improves skin texture and fills in scars and is contributing to the scar treatment market's projected growth to over $52.8 B by 2030.
REGENERATIVE THERAPY
Hair Restoration
Regenerating hair follicles, providing a novel solution for Alopecia.
Myocardium Restoration
Repairing heart tissue post-heart attack.
Nervous System Rebuilding
Restoring neural pathways, heralding breakthroughs in SCI and TBI treatment.
Skin Rejuvenation
Revitalizing aging skin, offering a natural route to youthfulness.
Wound Healing
Accelerating the healing of wounds, including diabetic foot ulcers.
Cartilage Repair
Repairing damaged cartilage, creating new hope for osteoarthritis patients.
Today, fat remains grossly underutilized.
Presently, four primary pain points prevent the efficient and effective use of fat in medical applications.
-
-
Limited Volumes
-

-

-
Labor Intensive
-
-
Slow Processing
-
Unpredictable Grafts
Sayenza Bio integrates solutions to these four challenges into a unified platform. Our platform is the key to a new era in fat utilization.
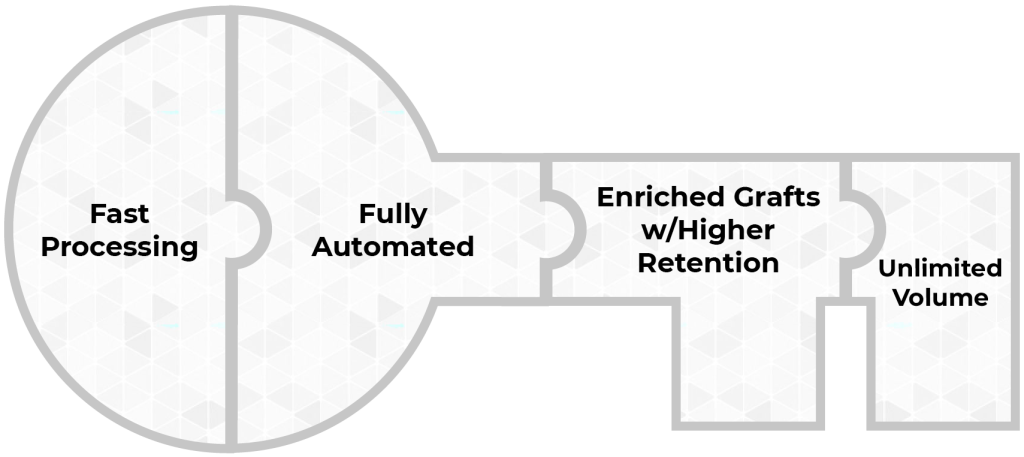
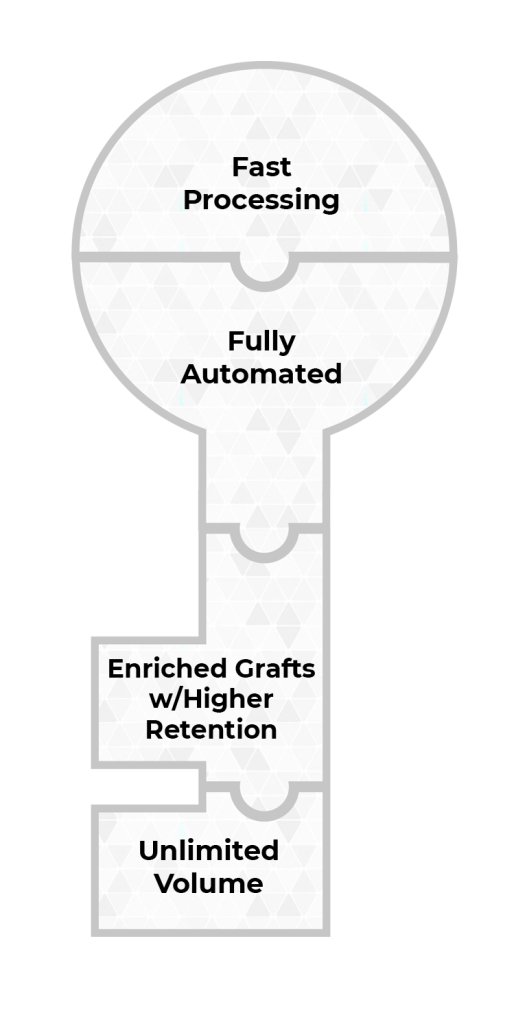




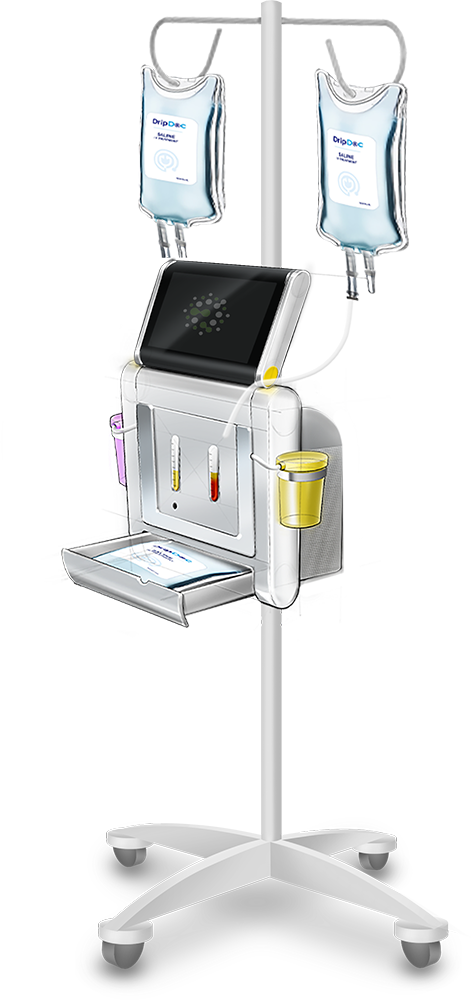
Automated processing w/ Regenerative Cell enrichment


Automated processing w/ Regenerative Cell enrichment.
Fully Automated
Reusable Platform
Self-Contained
High Precision


Dr. Ethan Kellum
Orthopedic Surgeon, Stem Cell Expert, Team Doctor: Tennessee Titans & USA Basketball


Drs. Alex Verpaele & Patrick Tonnard
World-Renowned Plastic Surgeons Inventors, Nanofat


Dr. Steven Williams
Plastic Surgeon, Tri Valley Plastic Surgery President, American Society of Plastic Surgeons
Backed by:















Get In Touch With Us Today
For any inquiries or information regarding our innovative regenerative medicine technologies, please contact us through our email info@sayenza.com.


















